Approved: Fortect
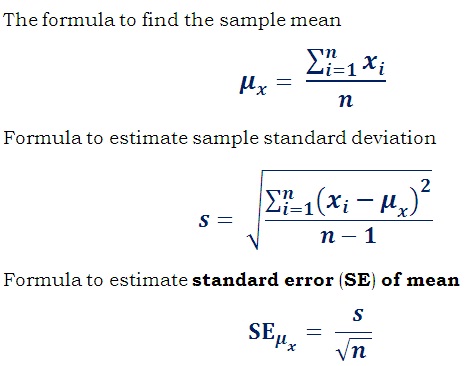
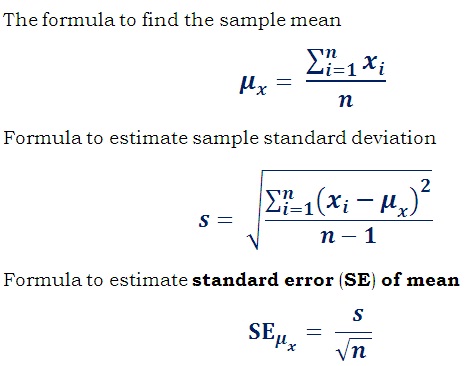
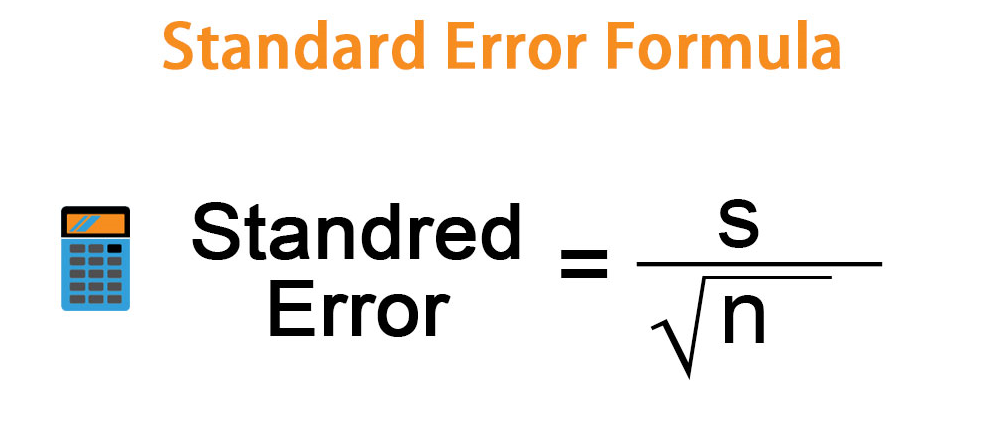
Hope this guide can help you if you have a rough estimate of the standard error on your system. The standard error is determined by simply dividing the standard deviation by the square root of the sample size. It indicates the accuracy of the model mean given the sample-to-sample variation in the sample mean.
Latent high temperatures are a form of internal and potential energy that is stored in evaporated or dissolved water. When the ice melts or ordinary liquid tap water evaporates, the molecules change state – from solid to liquid, from new liquid to gas, or from the best solid directly to gas.
The heat of fusion is the amount of heat energy required to change the physical state of a given substance from solid a to help you with liquid . It is also known as enthalpy synthesis. These are usually units in joules per gram (J / g) or calories per gram (cal / g). This example task shows how to calculate the basic amount of energy required to melt a real water sample from ice.
An Example Of A Problem
How much heat, in joules, does it take to melt 25 grams of a glacier? What are the calories in calories?
It is useful to understand: the heat of fusion of water corresponds to 334 J / g = 80 cal /
Solutions
ï ”¿Combined heat is applied during exercise. This is not a number that you need to know by heart. There are chemical tables that experts say give general thermal fusion values.
How do I download Adobe Flash Player on my PC?
To solve this problem, you need a formula that often associates thermal energy with the mass and heating of the fusion source:
q = = m · Î “H f
where
q thermal energy
m means mass and Î ”H f = heat of fusion.
It is clear that temperature does not appear in the equation, because what follows below does not change when the state of matter changes. The equation is simple, so it is important to make sure that you use the unit “yes” for the answer.
Approved: Fortect
Fortect is the world's most popular and effective PC repair tool. It is trusted by millions of people to keep their systems running fast, smooth, and error-free. With its simple user interface and powerful scanning engine, Fortect quickly finds and fixes a broad range of Windows problems - from system instability and security issues to memory management and performance bottlenecks.

To find heat in joules:
q = (25 g) x (334 J / g)
q equals 8350 J.
It is also easy to express heat in calories:
q means m · Î ” H f
q = G) x (80 (25 cal / g)
q = 1999 cal.
Answer: The amount of heat needed to thaw 25 grams of ice is equivalent to 8.350 joules or 2,000 calories.
Is latent heat of fusion melting?
Two common places where latent heat is accumulated are latent heat, which is generated by melting (melting), and latent heat, which is obtained by complete evaporation (boiling). These names describe the direction of the flow of energy in the transition from one movement to another: from solid to molten and from liquid to gas.
Note: the heat of fusion must be positive. (Helium is an exception.) If you receive a negative lot, please check your calculations.
Key Takeaways: Heat Of Fusion To Ice Melting
What is the latent heat of fusion for ice?
Latent heat of ice melting is 33600 J / K. Latent heat of ice melting is a new amount of heat required to melt a new unit of mass Ice from which a solid becomes liquid.
- Heat of fusion is a special amount of energy in the form of heat that is required to change the state of a material from solid to aqueous (melting).
- Formula for calculating the heat of the mixture: q = m · Î ”H f
- Note that the water temperature is not actually changingThey occur when the material improves, i.e., is not included in the method or calculation.
- Besides the release of helium, thermonuclear fusion always has a positive meaning.
or less so that the temperature is not too high hereDifference between water and air) in expanded polystyreneCup. We keep hot water in a suitable polystyrene cup.Minimize the loss of temperature rise to cool the area. A.Record the actual amount of water added so you can measure the cup.and record the hot water temperature just before your organization is readyadd ice.
Add one or two cubes (or about 1/4 cup Siamese crushed ice) to hotwater and gently stir with a thermometer until the winter storm is overmolten. Make sure you only add ice and not mains water.Place the dissolved ice in a bowl. When ice is in slicesmelted, measure and record the water temperatureTemperature almost dropped to freezing point or when not allowed to meltBecause of the ice it is necessary to return the impressions. you’llyou need to spend less money on Eating or more water, or both.
Use the master cylinder to measure the new volume of water.glass made of polystyrene. This new volume is compared withInitial volume as it contains water on thawed iceSince water has a content of 1 gram / ml (there is 1 gram / cm3), we are onthe relationship between measuring the volume of water in ml and the fact thatThe mass of the water itself in grams, the difference between the initial value andThe final volume of water is the mass of previously melted ice.
Can I download Adobe Flash Player for free?
Repeat the experiment at least one more time with a different amount.Water, many types of ice, and possibly a new starting temperatureWater.
Analyzing Data Conditions
when examining the actual test fromAn experiment is shown below. Initial is the thermal rangeWater water before adding ice, MWwater is the mass of the part (noteWater is actually diagnosed by measuring the initial massVolume of each water). We assume that ice will initially holdTemperature from
How do you determine latent heat of fusion of ice by the method of mixture?
The calorimetric stirrer is removed and weighed.Some liquids are poured into the calorimeter, then the mass and temperature of the calorimeter, stirrer, and water are measured.Take a small piece of ice and take into account the temperature, say (0 o C).
How do I download the latest Adobe Flash Player?
Does Adobe Flash Player 32 still work?