Approved: Fortect
If you can see on your computer why most metals are not found as elements on Earth, you need to check out these repair tips. Many metals can withstand natural weathering processes such as oxidation, so only the least reactive metals, such as gold and platinum, are usually present as natural metals. Metals are often extracted from the earth and also by mining, resulting in ores that are relatively rich sources, most often associated with essential elements.
Because of their reactivity, they tend to remove metal oxides as soon as they are exposed to air. Only metals that occupy high positions in the series of reactivity as a rule do not do this spontaneously, for example, gold.
Explanation:
Metals are rich in electrons and are therefore suitable for remanufacturers. Many metals occur naturally in the form of oxides or even sulfide ores.
Where are metals found on Earth?
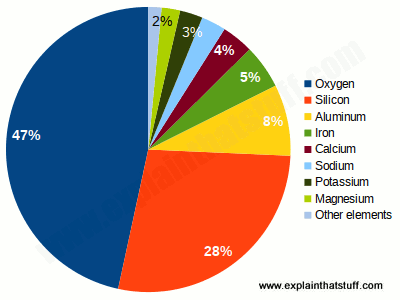
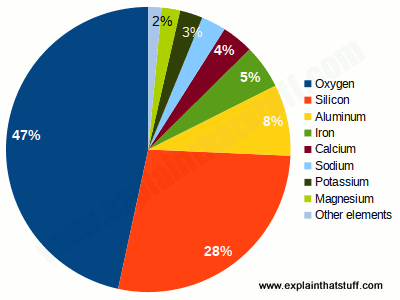
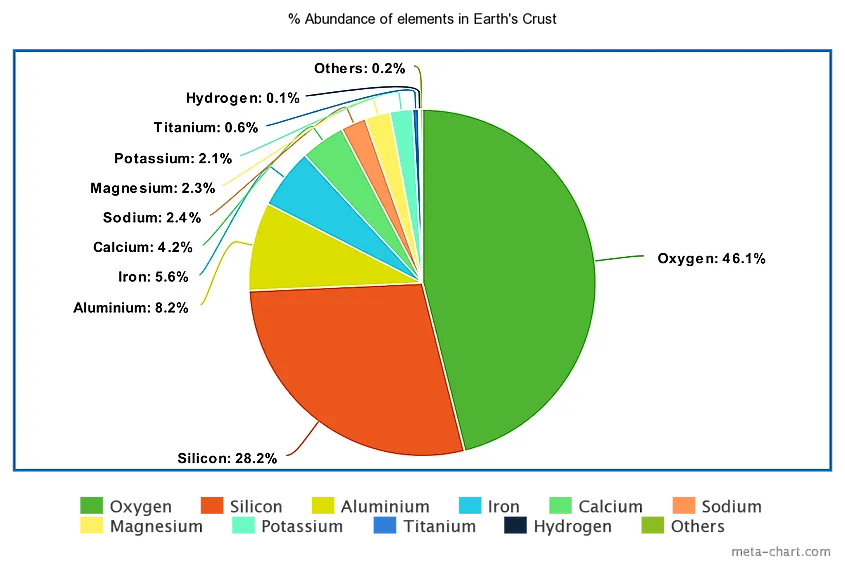
Many of these metals on Earth, especially iron, must be in its core. In fact, the metal is unevenly distributed in the earth’s crust, mixed with rocks, bound by oxygen and other elements. Some rocks, such as granite, actually contain only traces of metal.
One of the few components that are not reactive is jewelry, which can (still) be found as pure metal. And that’s how the golden element was connected on 2 continents centuries ago.
Presence Of Metals
Why are most metals found only in compounds?
Because of this reactivity, they tend to form chromium-effect oxides in the open air. Only metals that are lower in the series of reactivity, such as gold, are spontaneously prone to this.
Most pure metals are either too soft or brittle, or possibly reactive for practical use, one and a small amount of pure metals are allare traced in nature.
Native Metals
Native light aluminum is any metal present in its natural metallic form, either in its entirety or as an alloy. Metals that can be found individually and/or as alloys as deposits include antimony, arsenic, bismuth, cadmium, chromium, cobalt, indium, iron, nickel, selenium, tantalum, tellurium, tin, titanium, and zinc. >
Why are most metals not found in pure form?
Explanation Metals are rich in electrons and are good reducing agents. Many non-toxic metals are in the form of oxides or sulfide ores. One of the few metals that is not as reactive is gold, which can be found as a (still) pure metal.
You can also find two metal users: the Gold group and the American Eagle platinum group.
Why are all metals not found as metal ores?
Ores are a mixture of substances containing metals. Therefore, this is It can’t be a specific metal. Ores are made up of minerals, and this metal, of course, can also be profitably mined. Thus, all ores are minerals, but minerals whose metals simply cannot be mined in pure form are, of course, not ores.
Only silver, gold, silver copper and metals are found in nature in greater quantities. On a geologic timescale, very few materials can withstand natural weathering processes such as corrosion. For this reason alone, less sensitive metals such as gold and platinum are saw blades than native metals. Others mostly look like isolated pockets, a common chemical process duringSets the connection to normal or metal connections. Behind it is just pure metal, formed by small scales or inclusions, perhaps.
Approved: Fortect
Fortect is the world's most popular and effective PC repair tool. It is trusted by millions of people to keep their systems running fast, smooth, and error-free. With its simple user interface and powerful scanning engine, Fortect quickly finds and fixes a broad range of Windows problems - from system instability and security issues to memory management and performance bottlenecks.

Native metals were the only metals found in prehistoric humans. It is said that the process of extracting alloys from their ores (called smelting) began around 6500 BC. was opened. However, these metals can only be found in relatively small quantities so they cannot be widely used. Although photographer and iron were known long before the age of copper and iron, they could not have had much of an impact on humanity until the technology came along to allow them to be smelted on their ores and thus mass-produced. Alloy presents
Alloys
a mixture of two or more elements of a solid solution, the main component of which is metal. Most pure metals are too soft, brittle, or chemically reactive for practical use. The combination of different proportions of metals in the form of alloys changes the properties of pure metals to obtain the desired properties. Purpose manufacturedThe aim of other metals is usually to make the metals softer, more brittle, or more resistant to decay, or their color and improvement. brilliant.
Why do most metals exist in nature as minerals?
Most of the other metals are in the form of ores and even compounds containing oxygen and/or silicon, although they are reactive. Gold and silver treasures are relatively inert (especially gold) and are also found in their pure form.
Of all the alloys used, iron metal alloys (steel, stainless cast iron, cast iron, tool steel and mixed steel) account for the largest share, both in terms of simply commercial quantity and value. medium-low- and high-carbon steels are sufficient reasons for the different percentages of carbon alloyed with iron; Increasing the level of CO2 reduces ductility and toughness. The combined addition of silicon results in cast iron, and the addition of all chromium, nickel and molybdenum results in carbon steels (over 10%) of stainless steel.
Speed up your computer's performance now with this simple download.Why are most metals found in nature as ores?
All items have a durable beautiful outer shell. Therefore, most metals donate electrons to ionic compounds poet andalready present in nature in the form of ores.
Why are some metals not found in ores?
Only gold, copper, silver, and the platinum group are initially present in large amounts. Over geological time, very few compounds can withstand natural weathering processes such as corrosion, therefore less reactive metals such as gold and platinum occur mostly as natural metals.
Where are most metals found on Earth?
It is based on most of the metal base, in particular for hair straighteners. It is believed that the metal is unevenly distributed in the earth’s crust, mixed completely with the rock and associated with other elements and oxygen. Some rock types, such as granite, only contain traces of metal.